In today’s tightly regulated pharmaceutical industry, where the quality and safety of products directly impact public health, the importance of rigorous supply chain management cannot be overstated. With the global pharmaceutical market surpassing USD 1.48 trillion in 2022, the sector’s economic significance is undeniable. Yet, alongside its economic prowess comes heightened scrutiny from regulatory bodies and competition authorities, necessitating stringent compliance measures to uphold industry standards.
In this intricate regulatory landscape, the selection of qualified suppliers and the establishment of robust quality control mechanisms are paramount. However, as companies increasingly outsource and expand their supply chains, they face elevated risks and complexities. According to research from a 2020 Deloitte survey, 84% of respondents reported experiencing a third-party incident in the last three years. These incidents can result in financial repercussions, compliance challenges, and reputational damage.

Effective supplier management processes are essential for mitigating these risks. By synchronizing and integrating data with suppliers, companies can enhance collaboration, reduce speed-to-market times, and implement best practices and standardized processes. Moreover, such practices enable companies to identify which suppliers are most responsive, resilient, and aligned with their values.
However, without robust due diligence practices, it becomes challenging to ensure that suppliers meet regulatory requirements and maintain quality standards. Therefore, in this article, we will delve into the critical role of third-party due diligence in safeguarding the pharmaceutical industry, ensuring the integrity, quality, and regulatory compliance of pharmaceutical products throughout the supply chain.
What Is Third-Party Due Diligence?
Third-party due diligence is a critical process in the pharmaceutical industry, ensuring that potential suppliers meet stringent criteria such as regulatory compliance, good manufacturing practices, and quality standards. By performing due diligence, companies can mitigate risks and verify that suppliers are qualified to provide necessary materials or services.
In today’s global supply chains, sustainability risks are on the rise, with a significant surge in modern slavery and anticipated environmental costs reaching $120 billion for global companies by 2026. In the pharmaceutical industry, due diligence is paramount because the raw materials and services suppliers provide directly impact the quality and safety of final products. Without proper due diligence, pharmaceutical companies risk purchasing inferior or unsafe materials that could harm patients or lead to product recalls.
Vetting suppliers and vendors is essential in the pharmaceutical industry to safeguard the integrity and quality of medications. Partnering with reliable suppliers ensures access to high-quality materials and equipment crucial for producing safe and effective drugs. Vetting also helps identify potential compliance risks with regulations, preventing disruptions and ensuring adherence to strict industry standards. Additionally, it mitigates risks associated with supplier performance, financial instability, and unethical practices within the supply chain.
To achieve effective vetting, pharmaceutical companies scrutinize a supplier’s financial health, past compliance records, and Quality Management Systems. Reputation, security measures, and geographical location are also important factors to consider. By prioritizing experience in the industry and environmentally responsible practices, companies can build strong supplier relationships and ensure the overall quality and integrity of their products throughout the complex pharmaceutical supply chain.
Why Is Conducting Due Diligence of Third Parties Important in Pharma Supply Chains?
Conducting due diligence on third parties is crucial for managing risks effectively in pharmaceutical supply chains. By thoroughly vetting vendors, companies can identify potential risks early in the process, preventing costly mistakes and ensuring compliance with regulations. Third-party due diligence also helps uncover hidden risks such as poor environmental, social, and governance (ESG) practices or concentration risk, providing visibility into the supply chain ecosystem and enabling proactive risk mitigation strategies.
Supply chain due diligence is essential for pharmaceutical companies to uphold regulatory compliance, mitigate risks, and maintain ethical standards throughout their supply chains. Here are the key benefits specific to the pharmaceutical industry:
Regulatory Compliance:
Conducting supply chain due diligence ensures that pharmaceutical companies comply with stringent regulations imposed by health authorities, such as the FDA in the US and the EMA in Europe. This helps companies avoid legal penalties and regulatory actions while ensuring the safety and efficacy of their products.
Risk Mitigation:
By identifying and addressing risks within the supply chain, such as counterfeit drugs, product contamination, or supply disruptions, pharmaceutical companies can mitigate the potential impact on patient safety and business continuity. This proactive approach helps safeguard against financial losses and reputational damage.
Ethical Standards:
Supply chain due diligence enables pharmaceutical companies to verify that their suppliers uphold ethical standards, including fair labor practices, environmental sustainability, and human rights. By aligning with ethical principles, companies enhance their reputation, build trust with stakeholders, and contribute to a sustainable future.
Cost Efficiency:
Effective due diligence helps optimize supply chain processes, identify inefficiencies, and negotiate favorable terms with suppliers. This leads to cost savings, increased operational efficiency, and improved profitability for pharmaceutical companies.
Reputation Protection:
Maintaining transparency and integrity in the supply chain enhances the reputation of pharmaceutical companies and fosters trust among healthcare professionals, patients, and regulatory authorities. By prioritizing supply chain due diligence, companies demonstrate their commitment to quality, safety, and ethical business practices.
Overall, supply chain due diligence in the pharmaceutical industry is essential for ensuring regulatory compliance, mitigating risks, protecting patient safety, and upholding ethical standards. By investing in robust due diligence practices, pharmaceutical companies can strengthen their competitive position, safeguard their reputation, and contribute to the well-being of society.
Types of Due Diligence in Pharma Supply Chains
Regulatory Due Diligence:
This involves assessing compliance with regulatory requirements specific to the pharmaceutical industry, including adherence to Good Manufacturing Practices (GMP), Good Distribution Practices (GDP), and other relevant regulations enforced by health authorities.
Quality Due Diligence:
Quality due diligence focuses on evaluating the quality management systems and processes of suppliers to ensure the consistent production of safe and effective pharmaceutical products. This includes assessing quality control measures, documentation practices, and adherence to industry standards.
Security Due Diligence:
Security due diligence aims to identify and mitigate risks related to the security of pharmaceutical products throughout the supply chain. This includes assessing measures to prevent theft, counterfeiting, and tampering, as well as ensuring the integrity of transportation and storage facilities.
Ethical Due Diligence:
Ethical due diligence involves assessing suppliers' adherence to ethical standards, including fair labor practices, human rights, and environmental sustainability. This includes evaluating supplier policies on labor conditions, environmental impact, and social responsibility initiatives.
Product Safety Due Diligence:
Product safety due diligence focuses on ensuring the safety and efficacy of pharmaceutical products throughout the supply chain. This includes assessing manufacturing processes, product testing procedures, and measures to prevent contamination or adulteration.
By conducting thorough due diligence across these key areas, pharmaceutical companies can mitigate risks, ensure compliance with regulations, and uphold ethical standards in their supply chains.
Regulatory Imperatives for Supply Chains
Due diligence in the pharmaceutical industry transcends mere best practices; it has become a regulatory necessity. Several global acts and regulations underscore its critical importance, for instance, the US Foreign Corrupt Practices Act (FCPA) prohibits offering bribes to foreign officials for business gain, necessitating rigorous due diligence to ensure ethical sourcing practices across the pharmaceutical supply chain. Similarly, the EU Conflict Minerals Regulation targets conflict minerals funding armed conflict, setting a precedent for due diligence in raw material sourcing. The German Supply Chain Due Diligence Act (Lieferkettensorgfaltspflichtengesetz) mandates a risk management system to address human rights violations and environmental harm in supply chains, highlighting due diligence’s role in preventing corruption and upholding ethical practices.
These regulatory examples mirror a broader trend toward stricter supply chain due diligence regulations. Initiatives like the OECD Due Diligence Guidance for Responsible Business Conduct offer a globally recognized framework for managing social, environmental, and human rights risks in supply chains (Source: OECD).
While regulatory landscapes may vary by country, the emphasis on ethical sourcing and responsible supply chain management is increasingly a global priority. By implementing robust due diligence practices, pharmaceutical companies not only meet regulatory requirements but also showcase their dedication to ethical conduct and global patient welfare. A 2021 report by PwC projects that global spending on pharmaceutical track-and-trace solutions will reach USD 22.4 billion USD by 2025, emphasizing the substantial investment needed for compliance.
Navigating Risks in the Pharma Supply Chains
Operating within intricate market landscapes, the pharmaceutical sector faces unique challenges where demand is intertwined with public health dynamics and distribution networks are labyrinthine. This complexity exposes pharmaceutical companies to a myriad of risks, including bribery, corruption, and money laundering. Regulators caution against fraudulent schemes like transaction laundering, which exploit online payment channels to launder illicit funds through pharmaceutical product sales. Additionally, mergers and acquisitions pose another risk frontier, exemplified by Fresenius’ withdrawal from a deal with Akorn in 2018 due to data integrity breaches. The imposition of regulatory fines, amounting to billions of dollars, underscores the repercussions of due diligence failures, emphasizing the critical need for robust compliance measures. Multi-billion-dollar fines imposed on prominent companies like Eli Lilly, Pfizer, Merck, Johnson & Johnson, and Servier highlight the significant financial repercussions of regulatory and compliance transgressions. Heightened enforcement efforts across jurisdictions further underscore the risk landscape.
To navigate these challenges effectively, pharmaceutical businesses must strengthen their due diligence practices. Technology-driven solutions can streamline processes, enabling comprehensive risk assessment and compliance monitoring. By institutionalizing best practices and leveraging technology, companies can proactively address vulnerabilities, safeguarding their reputation and financial integrity amidst evolving regulatory pressures. Effective third-party risk and compliance management programs allow companies to protect their interests by identifying key risk exposures while maintaining innovation, market share, and profitability. Changing regulations and evolving technologies make it more challenging than ever to assess the risk profile of vendors, distributors, suppliers, and partners.
To safeguard the entire supply chain – from raw material procurement to product development and delivery to the consumer – comprehensive due diligence (CDD) checks are critical during vendor onboarding and throughout the entire contract lifecycle. Conducting due diligence during the vendor onboarding process helps identify risks posed by new partners, while ongoing research provides detailed information to make informed decisions about continuing business relationships. Doing business with a high-risk vendor can be detrimental to the bottom line, as enforcement actions by international regulators often result in costly penalties.
Challenges faced in Pharmaceutical Supply Chain Management
Organizations that lack effective external quality management capabilities, and instead use manual processes, may find themselves with gaps in communication, process delays, and potential product issues in the market. When this happens, it’s the stakeholder that faces the consequences, not the supplier. Managing an ecosystem of suppliers in this manner presents various risks to an organization.
Some of the more prevalent challenges to consider include the following:
Complex Nth-Party Relationships:
Managing numerous third-party relationships, including those of suppliers' suppliers (Nth parties), poses significant challenges due to limited oversight and visibility into this extensive ecosystem, leaving organizations vulnerable to various risks.
Supply Chain Disruptions:
Events like the COVID-19 pandemic highlight how disruptions, such as sourcing issues and transportation delays, can disrupt the pharmaceutical supply chain, underscoring the need for enhanced traceability and resilience.
Siloed Processes:
Operating in silos hampers collaboration and data sharing across departments, leading to fragmented insights and hindering proactive risk management efforts.
Limited Visibility:
Siloed supplier management processes result in inadequate visibility into the supply chain, making it challenging to anticipate and prevent supplier-related issues.
Onboarding Risks:
Effective supplier management should begin at the evaluation stage to identify high-risk suppliers before onboarding, ensuring proactive risk mitigation strategies are in place.
Inefficient Collaboration:
Manual processes impede data sharing and change tracking, leading to time-consuming and error-prone supplier collaboration, hindering operational efficiency.
Global Supply Chain Complexity:
Geographically dispersed supply chains with multiple stakeholders increase the risk of vulnerabilities and ethical lapses, necessitating robust due diligence practices to address transparency issues.
Emerging Threats:
The pharmaceutical industry faces rising threats such as counterfeit drugs, cyberattacks, and biosecurity concerns, highlighting the need for continuous adaptation of due diligence procedures and cybersecurity protocols.
Resource Constraints:
Comprehensive due diligence requires significant resources, including personnel, expertise, and technological tools, posing challenges for pharmaceutical companies, as many struggle to allocate sufficient resources for supply chain due diligence.
By addressing these challenges and implementing effective due diligence practices, pharmaceutical companies can enhance regulatory compliance, mitigate risks, and ensure the integrity and resilience of their supply chains.
Solutions for Effective Third-Party Due Diligence:
To effectively identify and evaluate supply chain and other third-party risks, companies require access to comprehensive, reliable information about their suppliers and vendors. Risk management and fraud prevention professionals need tools that enable them to anticipate risks occurring at any point in the extended global supply chain. However, relying solely on manual due diligence tools and consumer-oriented search engines proves to be costly and time-consuming.
Automated solutions are essential, conducting identity verification and flagging vendors for further investigation. Proactive risk intelligence tools can help organizations reduce exposure to corruption and fraud, ensure regulatory compliance with minimal disruption to the supply chain, mitigate reputational and regulatory risks, and maintain control over their supply chain partners.
Intelligent screening technology enables companies to:
- Gain in-depth knowledge of suppliers and safeguard against operational and reputational risks or sanctions.
- Develop a deep understanding of suppliers through ongoing adverse media searches of news and internet sources, corporate registration data, and litigation and regulatory databases.
,
- Detect and address critical risks in real-time by tracking a wide range of local, national, and international sources, including news media, trade journals, legal publications, and content not available on public websites.
- Easily understand relationships between parent and subsidiary companies, determine the level of risk associated with a person or business, and flag vendors for further investigation.
- Spot negative mentions about a vendor on social media platforms and assess potential negative affiliations with people and businesses.
Regtech companies that provide due diligence and compliance solutions for screening are valuable resources for companies seeking to enhance their third-party due diligence processes. These companies offer specialized tools and technologies designed to streamline due diligence, improve compliance, and mitigate risks associated with third-party relationships.
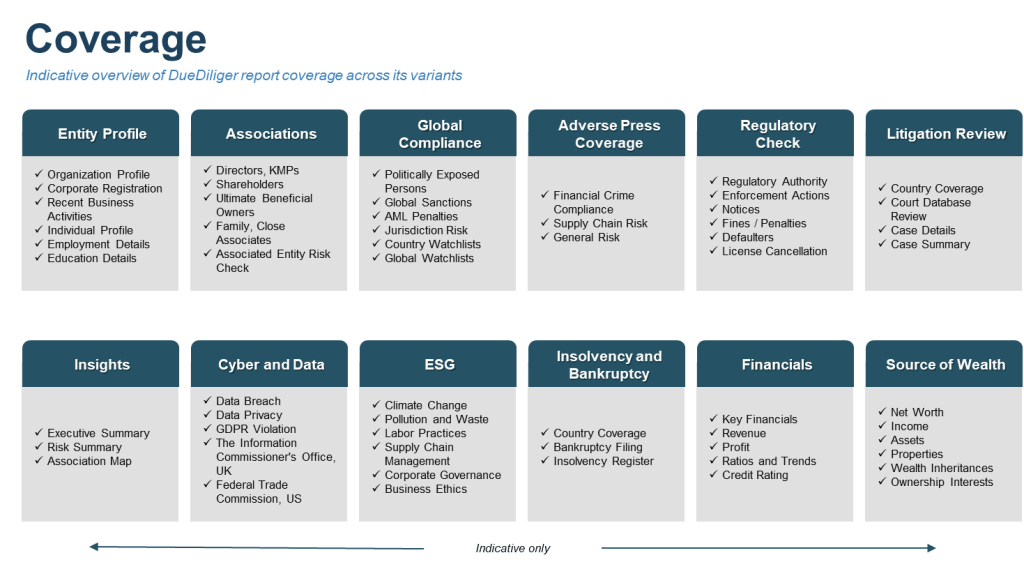
Zigram’s Comprehensive Due Diligence Reports for Mitigating Risks in the Supply Chains
Zigram’s Due diligence report solution offers deep, contextual and use case specific research to mitigate risks and fulfil regulatory requirements. Its features include-
- Multiple variants and types of reports
- Extensive coverage – 2,700+ watchlists and 7 Billion+ articles
- Specialist research and background check teams
- Technology enabled TAT and scale
In conclusion, the pharmaceutical industry's immense economic significance underscores the critical importance of robust supply chain management practices. With complex global supply chains come various risks, including regulatory fines and reputational damage. To address these challenges effectively, prioritizing third-party due diligence is essential. By thoroughly vetting suppliers, leveraging technology-driven solutions, and fostering collaborative initiatives, pharmaceutical companies can mitigate risks, ensure regulatory compliance, and uphold ethical standards throughout their supply chains. Additionally, partnering with reputable suppliers and embracing emerging technologies like blockchain and AI can further enhance due diligence efforts. Regtech companies specializing in due diligence and compliance solutions offer valuable resources to streamline processes and mitigate risks associated with third-party relationships. By investing in comprehensive due diligence practices, pharmaceutical companies can strengthen their competitive position, safeguard their reputation, and contribute to the well-being of society while ensuring the integrity and resilience of their supply chains.
- #Supply Chain
- #Due_Diligence
- #AML
- #Compliance